Pulmonary Function Testing Machines & Software Solutions
PFT solutions to improve your quality of care and grow your bottom line.
With KoKo PFT solutions, you (really) can have your cake and eat it, too.
Highest Accuracy
Fastest Results
Lowest Lifecycle Cost
Pulmonary function testing your patients, physicians, and CFO will love you for.
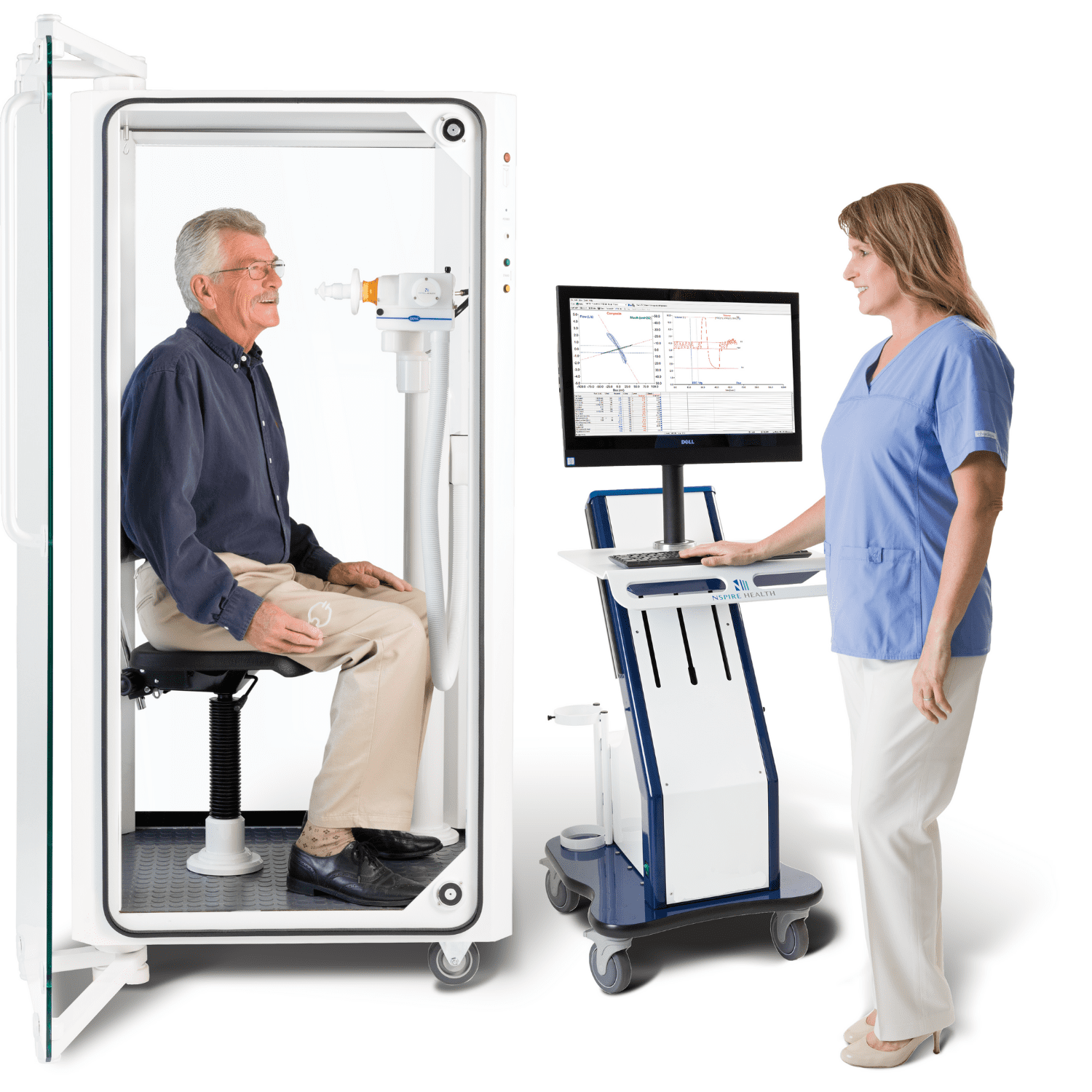
PFT MACHINES
KoKo Px delivers the highest quality, ATS-compliant pulmonary function test results in your modern, connected practice.
Spirometers
KoKo Sx 1000 gives you industry-leading performance with workflow maintenance and seamless EMR interoperability.
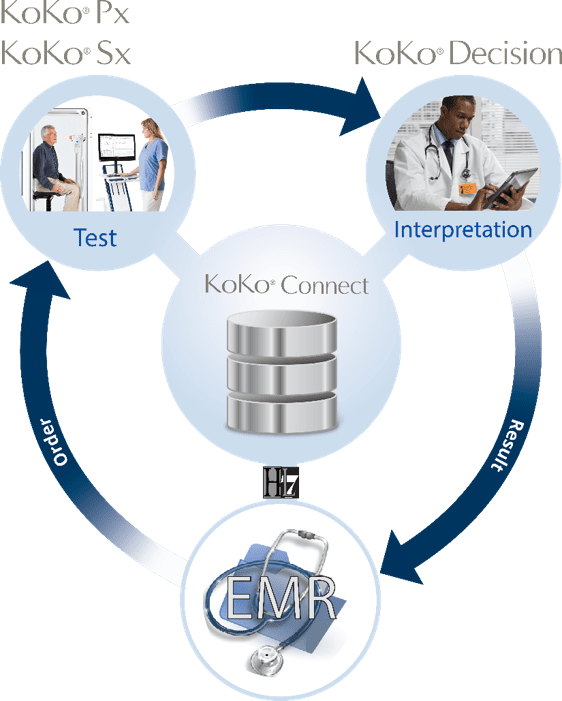
Experience the total PFT software solution
Why KoKo PFT software?
With KoKo Decision, your test results and interpretations are on all of your workstations
KoKo Connect syncs your test results and interpretations with your EMR—automatically
KoKo provided us with a deeper understanding of our lung function testing quality and cost drivers while offering our team focused training on corrective action procedures for more effective and efficient patient care. We saw instant results across facilities. –Sep. 2021
— Rodney Folz, M.D., PhD, Chief of Pulmonary, Critical Care, and Sleep Medicine at University Hospitals Cleveland Medical Center
You’re just one step away from knowing exactly how KoKo PFT devices & software will improve your quality of care and grow your bottom line.
Frequently asked questions
What test functions do KoKo spirometers and PFT machines offer?
KoKo spirometers can perform the following tests: Forced Vital Capacity, Slow Vital Capacity, Maximal Voluntary Ventilation, Challenge- Chemical & Exercise, and Pre- & Post- Bronchodilator Evaluation
KoKo Px comprehensive PFT machines can perform the following tests: Forced Vital Capacity, Slow Vital Capacity, Maximal Voluntary Ventilation, Challenge- Chemical & Exercise, Pre- & Post- Bronchodilator Evaluation, Diffusion Capacity- Single Breath & 3-EQ, Single Breath Methane Washout Lung Volumes, Maximum Inspiratory & Expiratory Pressure, Nitrogen Washout Lung Volumes- Multiple & Single Breath, Closing Volumes, Plethysmography Lung Volumes, and Airway Resistance & Specific Conductance.
What type of support do I get with KoKo PFT products (and are there any stipulations)?
Are KoKo’s software solutions compatible with existing IT connectivity and infrastructure?
Yes! As a software development company, KoKo ensures that its software solutions may be integrated into existing HL7 interface engines such as Allscripts, athenahealth, Cerner, Epic, GE Centricity, McKesson, MedFormix, Meditab IMS, Meditech, ModuleMD, NextGen, and more. This quality eliminates the need for a specialized interface and eases use for those familiar with their facility’s EMR system.